Can I Get H Pylori Again
- Research article
- Open Admission
- Published:
Long-term follow up Helicobacter Pylori reinfection rate afterwards 2nd-line treatment: bismuth-containing quadruple therapy versus moxifloxacin-based triple therapy
BMC Gastroenterology volume 13, Article number:138 (2013) Cite this article
Abstract
Background
The increasing trend of antibody resistance requires constructive 2nd-line Helicobacter pylori (H. pylori) treatment in high prevalence expanse of H. pylori. The aim of our written report was to evaluate the reinfection rate of H. pylori after second-line treatment that would determine the long-term follow upwardly effect of the rescue therapy.
Methods
A total of 648 patients who had failed previous H. pylori eradication on standard triple therapy were randomized into 2 regimens: 1, esomeprazole (20 mg b.i.d), tripotassium dicitrate bismuthate (300 mg q.i.d), metronidazole (500 mg t.i.d), and tetracycline (500 mg q.i.d) (EBMT) or 2, moxifloxacin (400 mg q.d.), esomeprazole (xx mg b.i.d), and amoxicillin (1000 mg b.i.d.) (MEA). At iv weeks after completion of eradication therapy, H. pylori tests were performed with 13C urea breath test or invasive tests. In patients who maintained continuous H. pylori negativity for the first yr after eradication therapy, H. pylori condition was assessed every yr. For the evaluation of risk factors of reinfection, gender, age, clinical diagnosis, histological atrophic gastritis or intestinal metaplasia were analyzed.
Results
The recrudescence charge per unit of the EBMT was 1.seven% and of the MEA group iii.iii% (p = 0.67). The annual reinfection rate of H. pylori of EBMT was found to be 4.45% and the MEA group half-dozen.46%. Univariate analysis (Log-rank test) showed no association with any clinical risk cistron for reinfection.
Conclusions
The long-term reinfection rate of H. pylori stayed low in both of bismuth-containing quadruple therapy and moxifloxacin-based triple therapy; thus reinfection cannot impact the choice of second-line treatment.
Trial registration
Clinical Trial Registration Number NCT01792700
Background
Helicobacter pylori (H. pylori) is a common pathogen of the gastric mucosa. It is estimated that at least fifty% of the world's human population has H. pylori infection [1]. Since the majority of patients with H. pylori infection practise non take whatever related clinical illness, routine screening is not considered [2]. However, equally the current prove suggests that H. pylori play a major role in peptic ulcer disease, gastric MALT lymphoma and in gastric cancer [three], screening and treatment in these diseases are recommended in several guidelines [2, iv–7]. In add-on, European guidelines recommend eradicating H. pylori infection in commencement-caste relatives of patients with gastric cancer, in long term NSAIDS or acid suppression users and in patients with functional dyspepsia [4]. Co-ordinate to these guidelines, public health efforts toward eradication volition be more effective in H. pylori high prevalence areas. Naturally, information technology is expected that increasing use of antibiotics must lead to increased resistance of antibiotics. Currently, the most ordinarily used initial handling is a triple regimen combining a proton pump inhibitor (PPI) with 2 antibiotics (clarithromycin and amoxicillin/or metronidazole) for the eradication of H. pylori[2, four–7]. Although this regimen has been shown to be effective in numerous clinical trials, the virtually recent data bear witness that the eradication rate has declined to less than 80% worldwide, largely related to development of resistance to clarithromycin [8]. In Korea, the recent eradication charge per unit of this regimen was less than lxxx% in a long-term follow upwards study (≥ 5 years) [nine, 10]. Therefore, this decreasing eradication charge per unit requires effective 2nd-line handling. Many clinicians have been using second-line therapy with bismuth-containing quadruple therapy or including fluoroquinolone antibiotics such as levofloxacin and moxifloxacin. In this situation, reinfection of H. pylori will determine the long-term effect of the eradication therapy for H. pylori. If a regimen shows a high reinfection charge per unit, then this eradication therapy should be avoided or strictly used only when absolutely indicated for H. pylori eradication. We reported the long-term annual reinfection rate of H. pylori in standard PPI-based triple therapy to be 3.51% per year in Korea [11]. Now that second-line therapy is ofttimes used in that location is increasing interest regarding the reinfection and recrudescence rates after rescue therapy. However, there are few reports regarding the reinfection rate of H. pylori later quadruple therapy [12] and none for quinolone based triple therapy. From this background the aim of our study was to evaluate the reinfection charge per unit of H. pylori afterwards two kinds of second-line treatment over a long-term follow up catamenia. In addition, we investigated the adventure factors for reinfection afterward this 2nd-line treatment.
Methods
Report population
The schematic flow of this study is shown in Figure 1. This was a prospective study performed betwixt 2003 and 2010 at Seoul National University Bundang Hospital in Korea. A total of 648 patients with persistent H. pylori infection after get-go-line treatment (PPI-based triple therapy) were enrolled. PPI-based triple therapy included PPI (standard dose), amoxicillin 1 thou, and clarithromycin 500 mg, all twice daily, for vii days. Patients were considered persistent H. pylori infection if 13C-urea breath test (UBT) or invasive H. pylori examination (Giemsa histology, CLO test, civilisation) were positive despite PPI-based triple therapy. Patients were excluded from the study if they had a history of renal or hepatic impairment, previous gastric surgery, pregnancy or lactation, therapy with steroids or non-steroidal anti-inflammatory drugs, or therapy with a proton pump inhibitor (PPI) or antibiotics within four weeks of entry. Between 2003 and 2006, the 44 patients with persistent H. pylori infection were treated with bismuth-containing quadruple therapy. Between 2007 and 2010, 604 patients with persistent H. pylori infection were randomized into 2 kinds of 2d-line therapy (bismuth-containing quadruple therapy or moxifloxacin-based triple therapy). Notwithstanding, if the patient preferred i regimen, after sufficient information for side event and eradication rate of each regimen a modify was permitted. Finally, 222 patients were treated for fourteen days with esomeprazole 20 mg b.i.d, tripotassium dicitrate bismuthate 300 mg q.i.d, metronidazole 500 mg t.i.d, and tetracycline 500 mg q.i.d (EBMT) as second-line treatment regimen for H. pylori infection. 426 patients were treated for 14 days with moxifloxacin 400 mg q.d, esomeprazole twenty mg b.i.d, and amoxicillin 1000 mg b.i.d (MEA) as second-line handling regimen for H. pylori infection. At four weeks after completion of the second-line handling, H. pylori eradication was evaluated by 13C-UBT or invasive tests. Invasive tests were performed in the patients in whom follow upward endoscopic examination was necessary for peptic ulcer, adenoma or gastric cancer. H. pylori negative status afterwards eradication was divers as a negative 13C-UBT or all negative of Giemsa stain, CLO test, and culture. Amongst 222 patients with EBMT eradication therapy and 426 patients with MEA eradication therapy, 169 patients and 308 patients were institute to be in eradicated status, respectively (Figure 1).
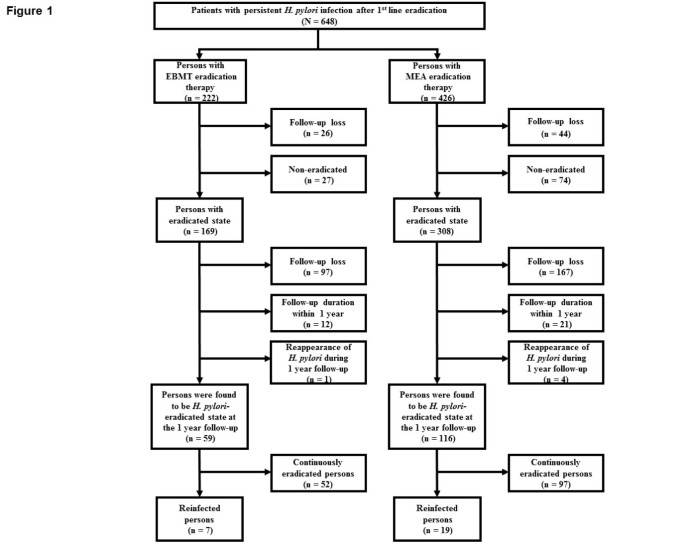
Schematic report catamenia chart.
All subjects provided informed consent, and the study protocol was approved by the Upstanding Committee at Seoul National University Bundang Hospital. ClinicalTrials.gov registration number is NCT01792700.
Invasive Helicobacter pyloritest (Giemsa histology, CLO exam, and civilisation) and histology
To determine the presence of electric current H. pylori infection, 10 biopsy specimens were taken from the gastric mucosa at each endoscopy (2 biopsy specimens each from the greater curvature of the antrum and body, and three each from the lesser curvature of the antrum and body). Among them, four biopsy specimens (i each from the greater curvature and lesser curvature of the antrum and trunk) were fixed in formalin, and used for determination of H. pylori infection by Giemsa staining. Another 4 specimens from the 4 gastric mucosa areas mentioned to a higher place were used for H. pylori culturing. The remaining two specimens from the lesser curvature of the antrum and body were used for the rapid urease test (CLO test; Delta West, Bentley, Australia).
Four of the biopsy specimens used for conclusion of H. pylori infection were also used for histological evaluation. These specimens were examined for the presence of gastric cloudburst and intestinal metaplasia past H&E staining. The presence of cloudburst on any of four specimens was diagnosed every bit gastric cloudburst, and the same method was applied to intestinal metaplasia. The definition of atrophy is the loss of appropriate glands including both metaplastic and not-metaplastic atrophy. Both metaplastic and not-metaplastic atrophy can be allocated to 1 of three grades of severity using grading criteria modeled on those suggested past the original and the updated Sydney Organization [thirteen].
thirteenC-urea jiff test
Patients fasted for four h earlier testing. Then, 100 mg of thirteenC-urea powder (UBiTkit; Otsuka Pharmaceutical, Tokyo, Nippon) was dissolved in 100 mL h2o and administered orally; a second breath sample was nerveless twenty min later. The collected samples were analyzed using an isotope-selective, non-dispersive infrared spectrometer (UBiT-IR300; Otsuka Pharmaceutical). The cutoff value used for H. pylori eradication was 2.five‰.
Follow-up of H. pyloritests
All of the eradicated patients received gastroscopy with invasive tests (modified Giemsa stain and CLO exam) non only from greater and lesser curvature of antrum but also from trunk after 1 year. If any one of these tests were positive then the patient was regarded as recrudescence case. Later on this time the patients were followed up for 1 yr with gastroscopy with invasive tests. Withal, when the patients preferred 13C-UBT or wanted to receive the gastroscopy every other year it was as well accustomed because the Korea government national wellness insurance program recommends biannual endoscopy instead of one twelvemonth.
Statistical analysis
The annual reinfection charge per unit (percentage per year) of H. pylori was calculated as (full number of infected patients/ cumulative observation years for all patients) 10 100.
SPSS for Windows (version xviii.0; SPSS, Inc., an IBM Company, Chicago, Illinois, U.s.a.) was used for all statistical analyses. Chiselled variables were analyzed using the Pearson chi-square test or Fisher's verbal test, and continuous variables were analyzed using independent samples t-exam. The hazard of H. pylori reinfection with fourth dimension was estimated using the Kaplan-Meier method. To make up one's mind the chance factors for reinfection, nosotros used the log-rank test. Nix hypotheses of no difference were rejected if p-values were less than 0.05.
Results
Patient characteristics
Amid eradicated 169 patients in the EBMT group and eradicated 308 patients in the MEA group, 59 patients and 116 patients maintained H. pylori-negative status continuously for ane yr, respectively (Figure 1). Specifically, 110 patients dropped out in the EBMT group and 192 in the MEA group for the post-obit three reasons: 97 patients in the EBMT group and 167 in the MEA grouping for not returning for gastroscopy or 13C-UBT later handling, 12 in the EBMT group and 21 in the MEA group for follow-up duration within 1 year, 1 patient in the EBMT grouping and 4 in the MEA group for reappearance of H. pylori during 1 year follow-upward. Finally, 59 patients and 116 patients in each group maintained H. pylori-negative continuously at one twelvemonth. The demographic and clinical characteristics of two report groups, who maintained H. pylori-negative continuously at i yr after the EBMT or MEA therapy, are summarized in Table one. Gender, the mean age of the patients, clinical diagnosis, atrophic gastritis, and intestinal metaplasia of the two groups were like. The enrolled early gastric cancer patients were cured by endoscopic submucosal dissection and follow-up was continuously performed regularly. During long-term follow-up patients in the EBMT or in the MEA group were divided into two groups: reinfected group and continuously eradicated grouping. The demographic and clinical characteristics of the reinfected and continuously eradicated group are summarized in Table 2. In the EBMT group and MEA grouping, there was no significant evidence that reinfection of H. pylori was related with gender, the hateful age of the patients, clinical diagnosis, atrophic gastritis, and intestinal metaplasia. The H. pylori recrudescence and reinfection rates are shown in Tabular array 3. One patient in the EBMT grouping and iv patients in the MEA group, who were H. pylori positive again at 1 year follow-upwards, were assigned to recrudescence cases. The rate was calculated at ane.7% (ane/threescore) for the EBMT group and 3.3% (four/120) for the MEA group, and these percentages were non significantly dissimilar (p = 0.67). During long-term follow-up one yr afterwards eradication H. pylori reappeared in vii (11.9%) of EBMT group and in nineteen (sixteen.4%) of MEA group and these percentages were not significantly dissimilar depending on each rescue treatment (p = 0.43). Among the reinfected persons no one was belonged to the same household.
Long-term follow-up and reinfection rate
The hateful duration of follow-upwardly of 59 patients in the EBMT group and 116 in the MEA group was 31.nine months (range: 18–90 months) and 30.iv months (range: eighteen–59 months). The mean number of H. pylori tests per patient was found to be 2.05 tests for the EBMT group and ii.31 tests for the MEA grouping (Table four). Reinfection with H. pylori occurred in 7 of 59 patients of EBMT group (11.ix%) and in 19 of 116 patients of MEA group (16.4%) sporadically during the follow-up menstruation. The calculated full almanac reinfection rate was found to be 4.45% (7/157.17 patient years X 100) for EBMT and 6.46% (19/294.08 patient years 10 100) for MEA.
Risk factors for reinfection
When the reinfected group (northward = 26) and continuously eradicated group (north = 149) were compared in terms of demographic data and clinical characteristics, no statistical differences were found by univariate analysis (Log-rank test), in both groups (Table v). Specially, there was no significant evidence that reinfection of H. pylori is related with eradication regimen (p = 0.23) (Figure 2).
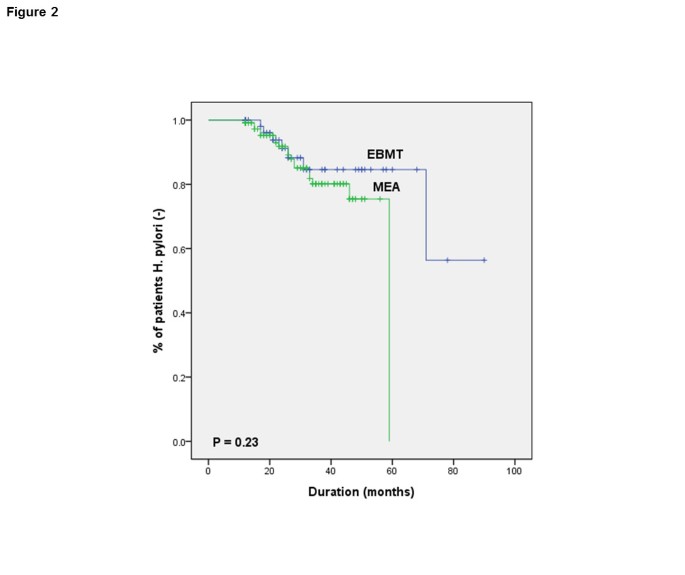
Kaplan-Meier curves for Helicobacter pylori reinfection according to regimen.
Give-and-take
We performed a prospective written report to investigate reinfection rate of H. pylori in patients who had been successfully treated with second-line therapy later an initial failure to eradicate H. pylori. To the best of our knowledge, this is the showtime report comparison the reinfection rate of EBMT and MEA therapy.
Reinfection is divers as an infection with a new strain of H. pylori that is dissimilar from the original strain after complete eradication, while recrudescence is a relapse of original strain, which was temporarily suppressed by eradication therapy [14, fifteen]. The recurrence rates of H. pylori subtract with fourth dimension and decline sharply later the first twelvemonth, and beyond the beginning year, recurrence rates come close to the rate of natural conquering of H. pylori infection in adulthood [fourteen, 16, 17]. From these reports the confirmation of continuous H. pylori negativity for the first year after eradication therapy has been accepted every bit consummate eradication [xviii–twenty]. Therefore, in our study, reinfection was defined equally the state of affairs where tests for H. pylori infection, later on continuous H. pylori negativity for the first year after eradication therapy, become positive again at a later phase. In addition, patients, who become H. pylori positive again during 1 year follow-upwards were classified every bit recrudescence cases. We could not perfectly distinguish between recrudescence of an original strain and reinfection because Deoxyribonucleic acid analysis of the strain using molecular fingerprinting techniques was not performed. Yet, this definition is supported past data obtained using Deoxyribonucleic acid analysis that the cause of H. pylori recurrence later first year is reinfection [fourteen].
In the previous study, we reported that annual reinfection in patients received standard PPI-based triple eradication therapy in Korea was 3.51% and the recrudescence rate 4.9% [11]. This result was similar to the hateful annual reinfection rate (3.iv%), calculated from the studies performed in adult countries [16]. The increase in antimicrobial resistance with the standard triple therapy has led to an increase of alternative therapy. However, there are few reports regarding the reinfection of H. pylori in patients received second-line therapy. In 2006, our group reported the annual reinfection charge per unit after second-line therapy (EBMT) during 1996–2004, at six.0% per patients-years in Seoul, Korea [12]. In the present long-term follow-upwards written report for up to 90 months we investigated the reinfection rate of EBMT and MEA therapy, performed during 2003–2010 in Gyeonggi province near Seoul, and those rates are 4.45% for EBMT and six.46% for MEA per yr. When eradication has truly been successful, reinfection is associated with the risk of re-exposure to H. pylori. Relatively low reinfection rates might exist related to the decrease in prevalence of H. pylori infection [21] and the recent improvement of sanitation conditions in Korea. In addition, when the reinfection rate of these ii kinds of rescue therapy were compared, there was no pregnant difference (p = 0.43). Therefore, we suggest that reinfection cannot bear on the choice of 2nd-line treatment.
In our study, recrudescence rate in the EBMT grouping and MEA group was found to be 1.vii% and 3.three%, which appears slightly lower than reported later initial eradication with standard PPI-based triple regimen (iv.nine%) [11]. This result might be related to the decreasing trend in eradication rate of standard triple therapy in Korea. That is, the eradication charge per unit of per protocol (PP) analysis decreased upwardly to 75.9% in 2006 [nine]. Some studies reported that the recurrence of H. pylori infection more frequently occurred in patients treated with a low efficacy regimen than in those treated with a high efficacy regimen, equally a issue of recrudescence of the organism after temporary suppression, not elimination [14, 16, 22, 23]. In the previous studies, the PP eradication rates were reported at 77.2% for the 7- and 93.6% for the xiv twenty-four hour period EBMT regimen [24], and 83.8% for the vii-, 82.6% for the 10- and 79.9% for the 14 day MEA regimen [25]. Our lower recrudescence rate might be related to the college efficacy of the EBMT and MEA regimens. In improver, there was no difference in the recrudescence rate between EBMT and MEA regimen (p = 0.67).
Limited data exists regarding risk factors for reinfection of H. pylori. Candidate run a risk factors include younger age [26, 27], infection of close contacts [14, 28], dental plaque [29, thirty], and contaminated endoscopic equipment [14, xvi, 31]. Other studies did non identify whatsoever factors predictive of H. pylori reinfection [32–34]. In the previous study, we reported that male gender and depression income were significantly associated with reinfection of H. pylori by multivariate analysis [11]. However, the current written report did not identify any predictive factors concerning H. pylori reinfection in the EBMT grouping and MEA group.
This study is the first study with large sample size and a long-term follow-upwardly menses in the investigation of reinfection rate of H. pylori in patients who had been successfully treated with second-line therapy after an initial failure to eradicate H. pylori. However, our study has limitations. First, recrudescence cases could be included in the reinfection cases. Theoretically the fingerprinting should exist performed for the differentiation of reinfected and recrudescence. However, we did not perform Dna assay to place the strains. In clinical practice, information technology is non easy to perform Deoxyribonucleic acid analysis. Secondly, despite our efforts to enroll all patients, many patients dropped out from this study, and refused to receive H. pylori tests every twelvemonth, especially when in that location was no gastrointestinal symptom.
Conclusions
In summary, in Korea, the long-term reinfection rate of H. pylori stayed low in both bismuth-containing quadruple therapy and moxifloxacin-based triple therapy; thus reinfection cannot affect the choice of second-line treatment.
References
-
Become MF: Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002, sixteen (Suppl 1): 3-xv.
-
Chey WD, Wong BC: American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007, 102: 1808-1825. 10.1111/j.1572-0241.2007.01393.10.
-
McColl KE: Clinical exercise. Helicobacter pylori infection. N Engl J Med. 2010, 362: 1597-1604. 10.1056/NEJMcp1001110.
-
Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al: Management of Helicobacter pylori infection–the Maastricht Iv/ Florence Consensus Study. Gut. 2012, 61: 646-664. 10.1136/gutjnl-2012-302084.
-
Fock KM, Katelaris P, Sugano K, Ang TL, Chase R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, et al: Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009, 24: 1587-1600. 10.1111/j.1440-1746.2009.05982.x.
-
Asaka K, Kato M, Takahashi Due south, Fukuda Y, Sugiyama T, Ota H, Uemura North, Murakami K, Satoh Chiliad, Sugano K: Guidelines for the management of Helicobacter pylori infection in Nippon: 2009 revised edition. Helicobacter. 2010, 15: ane-xx.
-
Kim North, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS: [Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea]. Korean J Gastroenterol. 2009, 54: 269-278. 10.4166/kjg.2009.54.5.269.
-
Graham DY, Fischbach L: Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010, 59: 1143-1153. 10.1136/gut.2009.192757.
-
Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim do H, Jung KW, Choi KD, Song HJ, Jung HY, et al: The trends of 1-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepato-gastroenterology. 2011, 58: 246-250.
-
Chung WC, Lee KM, Paik CN, Lee JR, Jung SH, Kim JD, Han SW, Chung IS: [Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study]. Korean J Gastroenterol. 2009, 53: 221-227.
-
Kim MS, Kim N, Kim SE, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, et al: Long-term Follow-up Helicobacter Pylori Reinfection Rate and Its Associated Factors in Korea. Helicobacter. 2013, xviii: 135-142. x.1111/hel.12018.
-
Cheon JH, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS: Long-term outcomes afterwards Helicobacter pylori eradication with second-line, bismuth-containing quadruple therapy in Korea. Eur J Gastroenterol Hepatol. 2006, 18: 515-519. x.1097/00042737-200605000-00010.
-
Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, Leandro G, Cost AB, Sipponen P, Solcia E, et al: Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002, sixteen: 1249-1259. 10.1046/j.1365-2036.2002.01301.x.
-
Zhang YY, Xia HH, Zhuang ZH, Zhong J: Review article: 'true' re-infection of Helicobacter pylori after successful eradication–worldwide annual rates, gamble factors and clinical implications. Aliment Pharmacol Ther. 2009, 29: 145-160. 10.1111/j.1365-2036.2008.03873.10.
-
Cameron EA, Bell GD, Baldwin L, Powell KU, Williams SG: Long-term study of re-infection post-obit successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006, 23: 1355-1358. x.1111/j.1365-2036.2006.02899.x.
-
Gisbert JP: The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am J Gastroenterol. 2005, 100: 2083-2099. 10.1111/j.1572-0241.2005.50043.x.
-
Peitz U, Hackelsberger A, Malfertheiner P: A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999, 57: 905-920. x.2165/00003495-199957060-00006.
-
Bell GD, Powell KU: Helicobacter pylori reinfection after credible eradication–the Ipswich feel. Scand J Gastroenterol Suppl. 1996, 215: 96-104.
-
Hildebrand P, Bardhan P, Rossi L, Parvin S, Rahman A, Arefin MS, Hasan G, Ahmad MM, Glatz-Krieger K, Terracciano 50, et al: Recrudescence and reinfection with Helicobacter pylori afterward eradication therapy in Bangladeshi adults. Gastroenterology. 2001, 121: 792-798. ten.1053/gast.2001.28018.
-
Soto G, Bautista CT, Roth DE, Gilman RH, Velapatino B, Ogura M, Dailide G, Razuri M, Meza R, Katz U, et al: Helicobacter pylori reinfection is mutual in Peruvian adults after antibiotic eradication therapy. J infect Dis. 2003, 188: 1263-1275. 10.1086/379046.
-
Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, et al: Seroprevalence of Helicobacter pylori in Due south Korea. Helicobacter. 2007, 12: 333-340. 10.1111/j.1523-5378.2007.00504.10.
-
Xia HX, Talley NJ, Keane CT, O'Morain CA: Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig Dis Sci. 1997, 42: 1821-1834. x.1023/A:1018827322470.
-
Seo G, Okada M, Shirotani T, Nishimura H, Maeda Thousand, Aoyagi K, Sakisaka S: Recurrence of Helicobacter pylori infection and the long-term outcome of peptic ulcer after successful eradication in Japan. J clin Gastroenterol. 2002, 34: 129-134. 10.1097/00004836-200202000-00005.
-
Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, et al: Bismuth-containing quadruple therapy equally second-line handling for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication charge per unit in Korea. Helicobacter. 2010, 15: 38-45. 10.1111/j.1523-5378.2009.00735.x.
-
Yoon H, Kim N, Lee BH, Hwang TJ, Lee DH, Park YS, Nam RH, Jung HC, Vocal IS: Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009, 14: 77-85.
-
Gomez Rodriguez BJ, Rojas Feria M, Garcia Montes MJ, Romero Castro R, Hergueta Delgado P, Pellicer Bautista FJ, Herrerias Gutierrez JM: Incidence and factors influencing on Helicobacter pylori infection recurrence. Rev Esp Enferm Dig. 2004, 96: 424-627.
-
Shimizu T, Yarita Y, Kaneko Grand, Yamashiro Y, Segawa O, Ohkura R, Taneike I, Yamamoto T: Case of intrafamilial Helicobacter pylori reinfection subsequently successful eradication therapy. Pediatr Infect Dis J. 2000, nineteen: 901-903. 10.1097/00006454-200009000-00024.
-
Gisbert JP, Arata IG, Boixeda D, Barba M, Canton R, Plaza AG, Pajares JM: Role of partner'due south infection in reinfection after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2002, 14: 865-871. 10.1097/00042737-200208000-00009.
-
Karczewska Due east, Konturek JE, Konturek PC, Czesnikiewicz Grand, Sito Eastward, Bielanski Westward, Kwiecien N, Obtulowicz W, Ziemniak West, Majka J, et al: Oral cavity as a potential source of gastric reinfection past Helicobacter pylori. Dig Dis Sci. 2002, 47: 978-986. ten.1023/A:1015017502772.
-
Kilmartin CM: Dental implications of Helicobacter pylori. J Tin Dent Assoc. 2002, 68: 489-493.
-
Sugiyama T, Naka H, Yachi A, Asaka M: Direct evidence by DNA fingerprinting that endoscopic cross-infection of Helicobacter pylori is a cause of postendoscopic acute gastritis. J Clin Microbiol. 2000, 38: 2381-2382.
-
Feydt-Schmidt A, Kindermann A, Konstantopoulos N, Demmelmair H, Ballauff A, Findeisen A, Koletzko S: Reinfection rate in children after successful Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2002, 14: 1119-1123. x.1097/00042737-200210000-00013.
-
Leal-Herrera Y, Torres J, Monath TP, Ramos I, Gomez A, Madrazo-de la Garza A, Dehesa-Violante Thousand, Munoz O: High rates of recurrence and of transient reinfections of Helicobacter pylori in a population with high prevalence of infection. Am J Gastroenterol. 2003, 98: 2395-2402. 10.1111/j.1572-0241.2003.07708.x.
-
Thong-Ngam D, Mahachai 5, Kullavanijaya P: Incidence of Helicobacter pylori recurrent infection and associated factors in Thailand. J Med Associ Thai. 2007, 90: 1406-1410.
Pre-publication history
-
The pre-publication history for this paper can be accessed hither:http://www.biomedcentral.com/1471-230X/13/138/prepub
Acknowledgments
This piece of work was supported by a grant from the National Research Foundation of Korea funded by the Korean Authorities (2012R1A1A3A04002680) and partly supported by the Seoul National University Budang Hospital Research fund (grants no 02-2010-014). MRCC of Seoul National University Hospital was consulted about the statistical assay of the present manuscript.
Author information
Affiliations
Respective author
Boosted information
Competing interests
The authors have no competing of interests to declare.
Authors' contributions
KN- designed the study and performed the major role of collecting patients; KMS- nerveless patients' data and wrote the manuscript; KSE- collected patients' data and was involved in editing the manuscript; JHJ- collected patients' information and were involved in editing the manuscript; SCM- collected patients' information and were involved in editing the manuscript; PYS- nerveless patients' information and were involved in editing the manuscript; LDH- collected patients' information and was involved in editing the manuscript. All authors read and approved the last manuscript.
Authors' original submitted files for images
Rights and permissions
This commodity is published under license to BioMed Primal Ltd. This is an Open Access article distributed nether the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/ii.0), which permits unrestricted utilise, distribution, and reproduction in any medium, provided the original piece of work is properly cited.
Reprints and Permissions
Almost this article
Cite this article
Kim, G.S., Kim, N., Kim, S.E. et al. Long-term follow up Helicobacter Pylori reinfection rate after 2d-line treatment: bismuth-containing quadruple therapy versus moxifloxacin-based triple therapy. BMC Gastroenterol 13, 138 (2013). https://doi.org/10.1186/1471-230X-thirteen-138
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1471-230X-13-138
Keywords
- Helicobacter pylori
- Reinfection
- Quadruple
- Moxifloxacin
- Second-line
Source: https://bmcgastroenterol.biomedcentral.com/articles/10.1186/1471-230X-13-138
0 Response to "Can I Get H Pylori Again"
Enregistrer un commentaire